Abstract
Background B-cell prolymphocytic leukemia (B-PLL) is a rare hematologic malignancy, accounting for <1% of all chronic B-cell leukemia cases, and is generally associated with aggressive features and poor clinical outcomes. Given its low incidence, there is limited data available regarding practice patterns and treatment outcomes in this population. We aimed to evaluate, on a national scale, the trends and overall survival outcomes of patients with B-PLL.
Methods The National Cancer Database was used to identify patients with B-cell PLL from 2004-2019. For patients receiving treatment, the use of single or multiagent therapy and upfront transplant was compared, with the utilization of immunotherapy identified for patients diagnosed 2013 or later, corresponding to the recoding of rituximab and alemtuzumab. Kaplan-Meier and Cox regression analysis was used to compare overall survival (OS) for all B-PLL pts. Survival was compared by year of diagnosis, with diagnosis in 2004-2014 considered to be prior to the availability novel therapies, 2015 and later associated with the introduction of BTK and PI3K inhibitors and 2017 as considered having access to venetoclax. Multivariate analysis was performed adjusting for age, co-morbidity score, facility type, and insurance status.
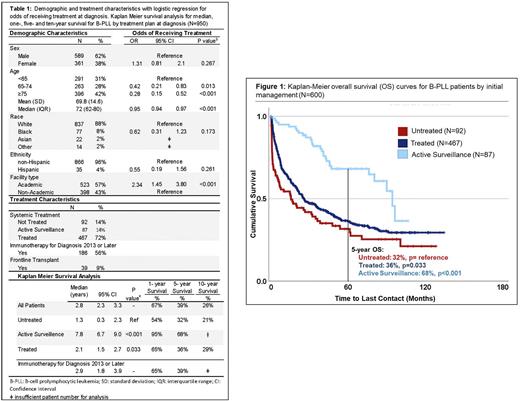
Results Of the 950 B-PLL patients identified, the median age of diagnosis was 72 years (interquartile range 62-80) with 42% of patients being ≥75 years old. The majority (88%) identified as White and were male gender (62%). For those with treatment data available (68%; N=646), the majority of patients (72%) received frontline systemic therapy at diagnosis, whereas 14% were managed with active surveillance, and 14% did not receive any form of treatment. Of the 467 patients receiving upfront treatment, 45% of these patients were treated with single agent therapy, 41% received multiagent chemotherapy, and 14% had an unspecified therapy. For those with transplant and immunotherapy data available, 186 patients received immunotherapy and 39 patients were treated with transplant in the front-line. B-PLL patients were more likely to receive treatment if managed at an academic center (OR 2.34; 95% CI 1.45-3.80, p<0.001), and less likely to receive treatment with each increased year of age (OR 0.95; 95% CI 0.94-0.97, p<0.001) or with increased comorbidity burden (OR 0.52 for Charlson-Deyo comorbidity score ≥2, 95% CI 0.28-0.98, p<0.001).
With a median follow-up of 1.5 years, B-PLL patients had a median OS of 2.8 years (95% CI 2.3-3.3), with a 1-, 5-, and 10- year survival of 67%, 39%, and 26%, respectively. Patients managed with upfront active surveillance had a median OS of 7.8 years (95% CI 6.7-9.0 years, p<0.001), and patients who underwent upfront treatment had a median OS of 2.1 years (95% CI 1.5-2.7, p<0.033), with a 1-,5-, and 10- year OS of 65%, 36%, and 29%, respectively (all p<0.03). Patients who did not receive disease directed therapy due to death, hospice care, or contraindication, had a median OS of 1.3 years (95% CI 0.3-2.3). For patients treated with immunotherapy and diagnosed 2013 or later (N=166), median OS was 2.9 years (95% CI 1.8-3.9). On multivariate analysis, factors associated with increased risk of death included increased age (Hazard Ratio [HR] 1.04, 95% CI 1.03-1.05) and higher comorbidity score (HR 1.40, 95% CI 1.27-1.54) (all p<0.001). When examining survival trends by year of diagnosis, patients diagnosed prior to BTK and PI3K inhibitor availability (N=646) had no statistically significant difference in OS compared to those diagnosed 2015 or after (N=149) with a median OS of 2.7 years vs. 3.4 years (p=0.432). Additionally, patients diagnosed 2017-2019, with availability of venetoclax (N=175), also had no significant improvement in median OS (2.6 years; 95% CI 1.7-3.5, p=0.885) referenced to diagnosis from 2004-2014.
Conclusions These data comprise the largest study to date evaluating patient outcomes with B-PLL. We found that this disease primarily affects elderly patients with a higher incidence in males compared to females. Though the incorporation of novel therapies in recent years has increased therapy options in B-PLL, these data show no significant improvement in overall survival. The continued poor outcomes of this aggressive disease suggests that there is still a high need for clinical research and novel therapeutic options for these patients.
Disclosures
Shah:AbbVie: Membership on an entity's Board of Directors or advisory committees; ADCT: Research Funding; BeiGene: Research Funding; Astrazeneca: Research Funding; Seattle Genetics: Research Funding; Epizyme: Research Funding. Hu:Bristol Meyers Squibb: Consultancy; Novartis: Consultancy; ADC Therapeutics: Consultancy; Lymphoma Research Foundation: Research Funding; Genentech: Research Funding; Celgene: Research Funding; CRISPR Therapeutics: Research Funding; Caribou Biosciences: Research Funding; Morphosys AG: Research Funding; Repare Therapeutics: Research Funding. Stephens:AbbVie: Consultancy; Novartis: Research Funding; Mingsight: Research Funding; Acerta: Research Funding; JUNO: Research Funding; Karyopharm: Research Funding; Arqule: Research Funding; Newave: Research Funding; Epizyme: Consultancy; AstraZeneca: Consultancy; TG Therapeutics: Consultancy; Lilly: Consultancy; Genentech: Consultancy; Celgene: Consultancy; Beigene: Consultancy; CSL Behring: Consultancy.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal